Data Analysis
Phase A
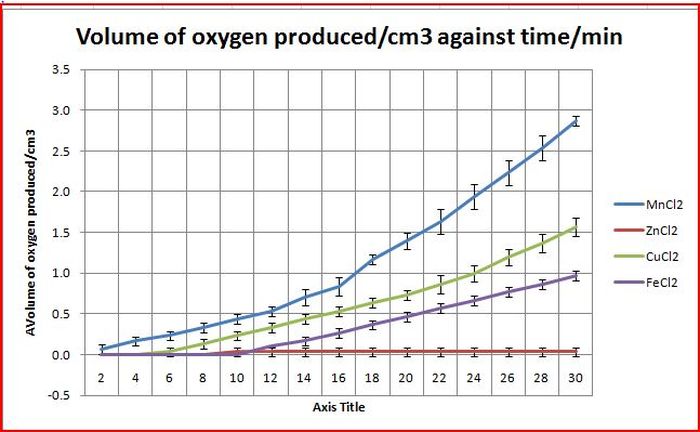
Objective: To find out which is the best transition metal salt among Copper(II) chloride, Zinc chloride, Iron(II) chloride and Manganese Chloride
From the graph above, although some other transition metal salts such as copper(II) chloride and iron(II) chloride were able to catalyze the disproportionation of hydrogen peroxide, manganese remained as the best catalyst for the reaction. Zinc(II) chloride was unable to catalyze the disproportionation of hydrogen peroxide within the 30 minute time frame.
This results prove that our hypothesis is only half true. Although we predicted that transition metals are able to catalyze the disproportionation of hydrogen peroxide because of the ability to change oxidation state and become a good catalyst, zinc chloride did not show the properties we predicted. However it could be noted that since zinc is at the side of the periodic table, it does not have strong transition metal properties and is unable to change oxidation state so quickly and easily, thus it could explain why zinc was unable to catalyze the disproportionation of hydrogen peroxide despite being a transition metal.
Manganese chloride was used for the subsequent experiments as it is the best transition metal and it could more effectively show how various factors affect the rate of disproportionation of hydrogen peroxide.
This results prove that our hypothesis is only half true. Although we predicted that transition metals are able to catalyze the disproportionation of hydrogen peroxide because of the ability to change oxidation state and become a good catalyst, zinc chloride did not show the properties we predicted. However it could be noted that since zinc is at the side of the periodic table, it does not have strong transition metal properties and is unable to change oxidation state so quickly and easily, thus it could explain why zinc was unable to catalyze the disproportionation of hydrogen peroxide despite being a transition metal.
Manganese chloride was used for the subsequent experiments as it is the best transition metal and it could more effectively show how various factors affect the rate of disproportionation of hydrogen peroxide.
Phase B
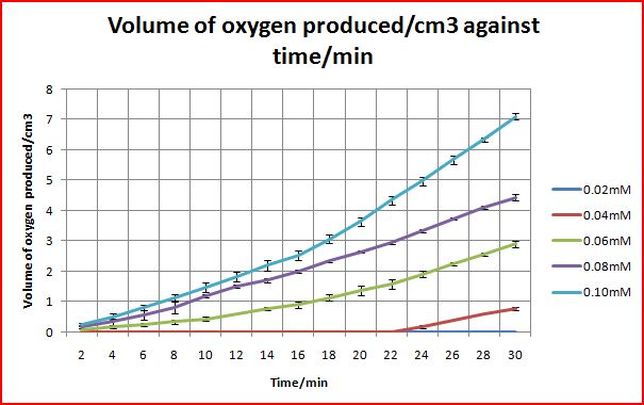
Objective: Find out how the concentration of the best transition metal salt affects the rate of disproportionation of hydrogen peroxide.
The concentrations would be varied five times: 0.02mM, 0.04mM, 0.06mM, 0.08mM, 0.10mM
The concentrations would be varied five times: 0.02mM, 0.04mM, 0.06mM, 0.08mM, 0.10mM
From the graph above, it shows that the higher the concentration of manganese chloride, the higher the rate of oxygen produced.
This also confirms that manganese chloride does act like a catalyst.
This also confirms that manganese chloride does act like a catalyst.
Phase C
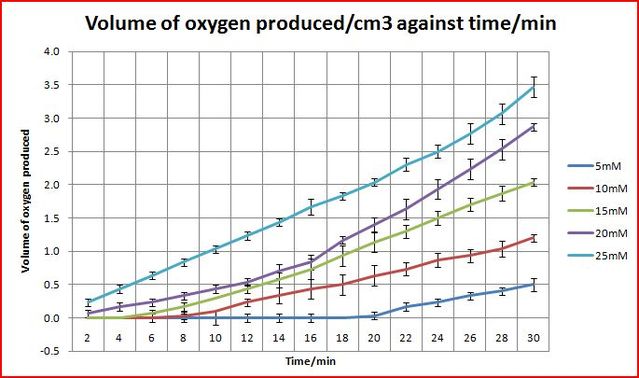
Objective: Find out how the concentration of hydrogen peroxide affects the rate of disproportionation.
The concentrations would be varied five times: 5mM, 10mM, 15mM, 20mM, 25mM
The concentrations would be varied five times: 5mM, 10mM, 15mM, 20mM, 25mM
From the graph above, it shows that with a higher concentration of hydrogen peroxide, a higher rate of oxygen produced.
Phase D
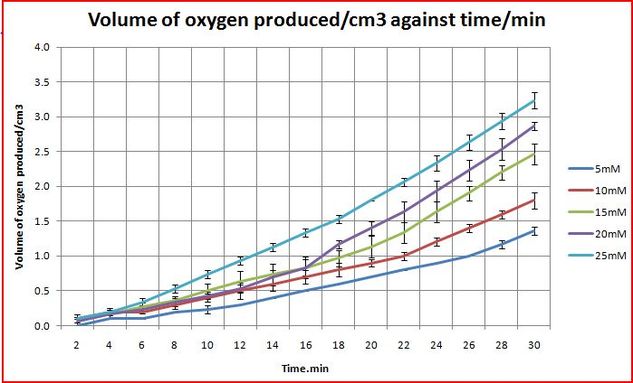
Objective: Find out how the concentration of sodium bicarbonate (pH) affects the rate of disproportionation of hydrogen peroxide.
The concentrations would be varied as follow: 5mM, 10mM, 15mM, 20mM, 25mM
The concentrations would be varied as follow: 5mM, 10mM, 15mM, 20mM, 25mM
Sodium bicarbonate increases the pH of the environment where the reaction take place.
From the graph above, in a higher concentration of sodium bicarbonate, in a higher pH, there is a higher rate of oxygen produced.
From the graph above, in a higher concentration of sodium bicarbonate, in a higher pH, there is a higher rate of oxygen produced.
Conclusion
Our results support our hypothesis that some of the transition metal salts besides manganese can catalyze the disproportionation of hydrogen peroxide. However not all are able to do so and this is evident in zinc chloride.
Transition metal salts are able to catalyze the reaction of disproportionation of hydrogen peroxide, manganese salt was still the best catalyst. It also tells us that in more concentrations of hydrogen peroxide, more concentrations of transition metal salt and in a more extreme pH, the rate of reaction is the highest.
Transition metal salts are able to catalyze the reaction of disproportionation of hydrogen peroxide, manganese salt was still the best catalyst. It also tells us that in more concentrations of hydrogen peroxide, more concentrations of transition metal salt and in a more extreme pH, the rate of reaction is the highest.